RML is proud to introduce RML LabWorks® Mobile for use with the Apple iPhone® or iPod® Touch®. Beneficial to physicians and other healthcare providers, RML LabWorks® Mobile gives active caregivers secure access to patient results from their mobile device - no matter where they are.
Quality care means access to clinical data 24 / 7. With RML LabWorks® Mobile, physicians will:
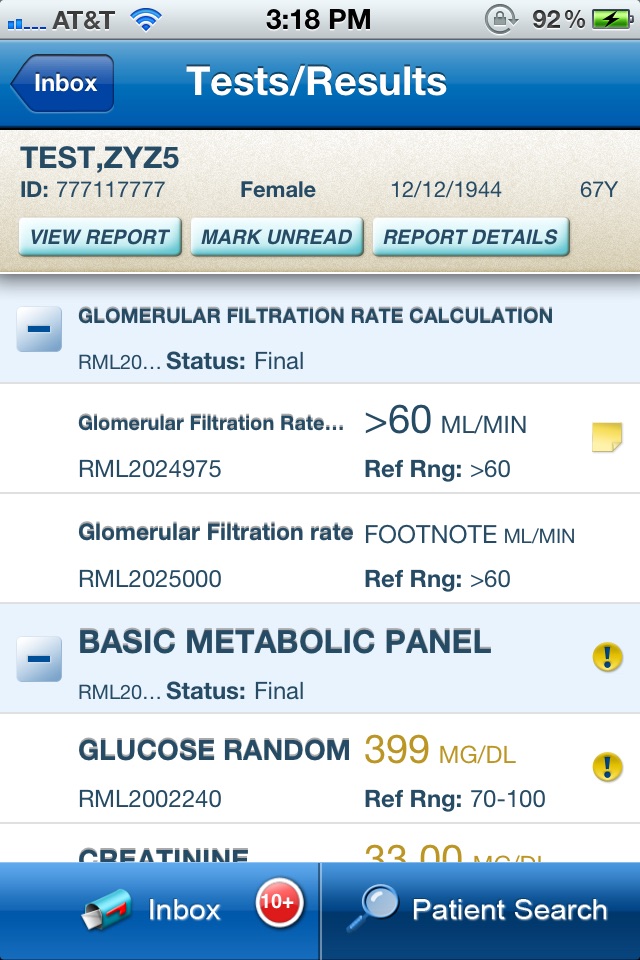
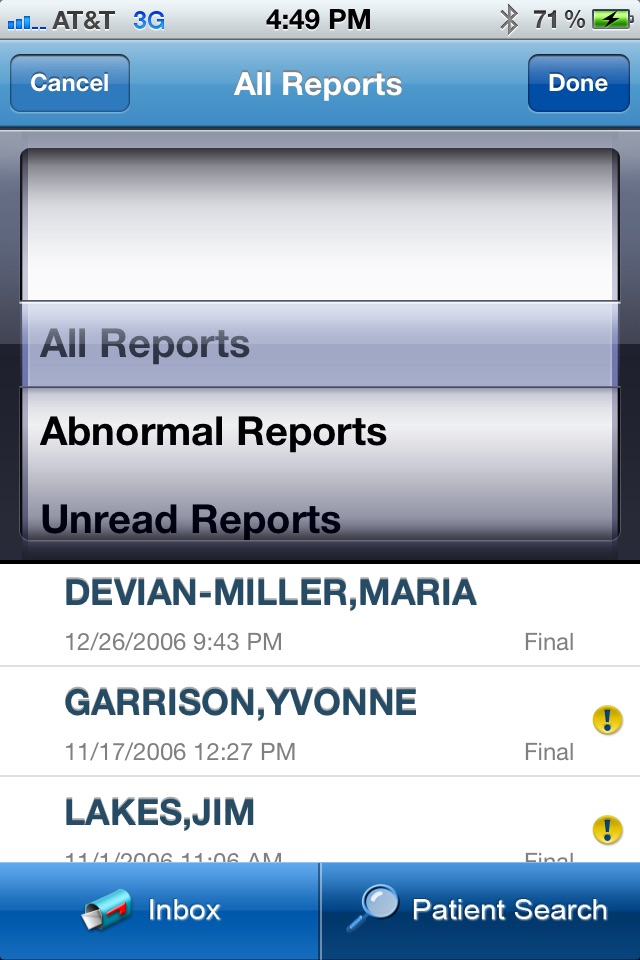
- View new / unread patient results, with abnormal result flagging
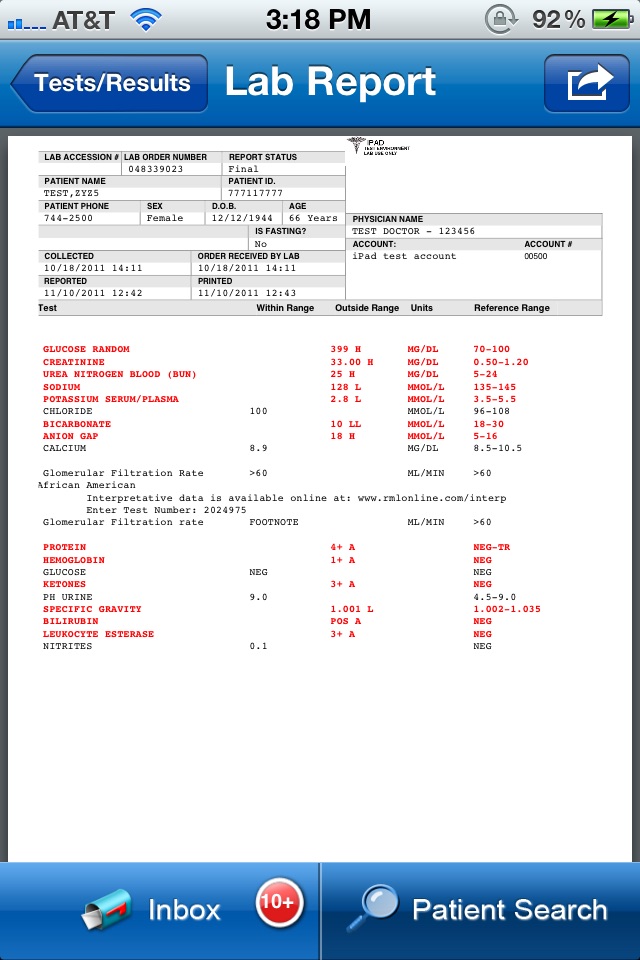
- Access complete patient reports, with interpretive notes
- Verify individual patient demographics to ensure patient safety
- View historical lab results
- Phone patient while viewing results with simple tap-to-call option
This application requires the physician be a registered RML LabWorks® user. If you are a user associated with more than one site, you will have the option to select applicable location at log in. If you are not a registered RML LabWorks® user, contact your laboratory representative to learn more about RML LabWorks® and RML LabWorks® Mobile.
With RML LabWorks® Mobile you can have comfort in knowing that where you lead, the RML LabWorks® Mobile application will deliver accurate patient results — anytime, anywhere. Download the app today and join the RML LabWorks® Mobile movement.
Regional Medical Laboratory, Inc. is a nationally-renowned commercial pathology laboratory that provides testing services for thousands of physicians and hospitals within a four-state region. We procure blood and urine samples from patients, evaluate tissue biopsies to determine whether a patient has cancer, perform blood tests to calculate cholesterol levels and rule out disease, perform autopsies to determine cause of death, complete drug and alcohol screenings and much more. The highest standard of quality is maintained through a vigorous quality assurance program including certification by the College of American Pathologists, the Oklahoma State Department of Health, the United States Department of Health and Human Services, and registration with the Food and Drug Administration.